
Varithena reimbursement and coverage
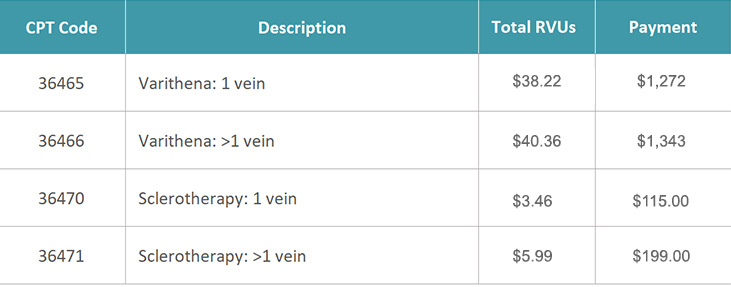
Varithena is classified by two dedicated Category I CPT® codes: 36465 and 36466
CPT codes help simplify your practice.
Dedicated CPT codes 36465 and 36466 can make your practice more efficient and allow for consistent and predictable reimbursement policies.
36465 - Injection of non-compounded foam sclerosant with ultrasound compression maneuvers to guide dispersion of the injectate, inclusive of all imaging guidance and monitoring; single incompetent extremity truncal vein (e.g., great saphenous vein, accessory saphenous vein).
36466 - Injection of non-compounded foam sclerosant with ultrasound compression maneuvers to guide dispersion of the injectate, inclusive of all imaging guidance and monitoring; multiple incompetent truncal veins (e.g., great saphenous vein, accessory saphenous vein), same leg.
2024 Medicare physician fee schedule final rule
Varithena may be billed with one of the following CPT codes listed below, for the physician office setting. Per CPT instructions, the code selected should accurately describe the service performed.
1. 2024 CMS Physician Fee Schedule. CMS-1784-F. Effective through December 31, 2024. Conversion factor $33.2875.
Reimbursement request
References
- Data on file. Provensis Ltd, 2013.
Disclaimer
Health economic and reimbursement information provided by Boston Scientific Corporation is gathered from third-party sources and is subject to change without notice as a result of complex and frequently changing laws, regulations, rules and policies. This information is presented for illustrative purposes only and does not constitute reimbursement or legal advice. Boston Scientific encourages physicians to submit accurate and appropriate claims for services. It is always the physician's responsibility to determine medical necessity, the proper site for delivery of any services and to submit appropriate codes, charges, and modifiers for services that are rendered. Boston Scientific recommends that you consult with your payers, reimbursement specialists and/or legal counsel regarding coding, coverage and reimbursement matters. It is always the physician's responsibility to understand and comply with national coverage determinations (NCD), local coverage determinations (LCD) and any other coverage requirements established by relevant payers which can be updated frequently.
CPT® Copyright 2020 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS Restrictions Apply to Government Use. Fee schedules, relative value units, conversion factors, and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein.
Indications
Varithena® (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena® improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
Important Safety Information
The use of Varithena® is contraindicated in patients with known allergy to polidocanol and those with acute thromboembolic disease. Severe allergic reactions have been reported following administration of liquid polidocanol, including anaphylactic reactions, some of them fatal. Observe patients for at least 10 minutes following injection and be prepared to treat anaphylaxis appropriately. Intra-arterial injection or extravasation of polidocanol can cause severe necrosis, ischemia or gangrene. Patients with underlying arterial disease may be at increased risk for tissue ischemia. If intra-arterial injection of polidocanol occurs, consult a vascular surgeon immediately.Varithena® can cause venous thrombosis. Follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization, or pregnancy are at increased risk for developing thrombosis. The most common adverse events observed were pain/discomfort in extremity, retained coagulum, injection site hematoma or pain, common femoral vein thrombus extension, superficial thrombophlebitis, and deep vein thrombosis.Physicians administering Varithena® must be experienced with venous procedures, possess a detailed working knowledge of the use of the duplex ultrasound in venous disease and be trained in the administration of Varithena®.
See Full Prescribing Information for Varithena®
Varithena™ is a registered trademark of Boston Scientific. All other trademarks are property of their respective owners.
PI-1263705-AA

