A low-nitrogen solution that leads to increased safety and performance.
.png)
Easy and efficient for you and your staff to use.
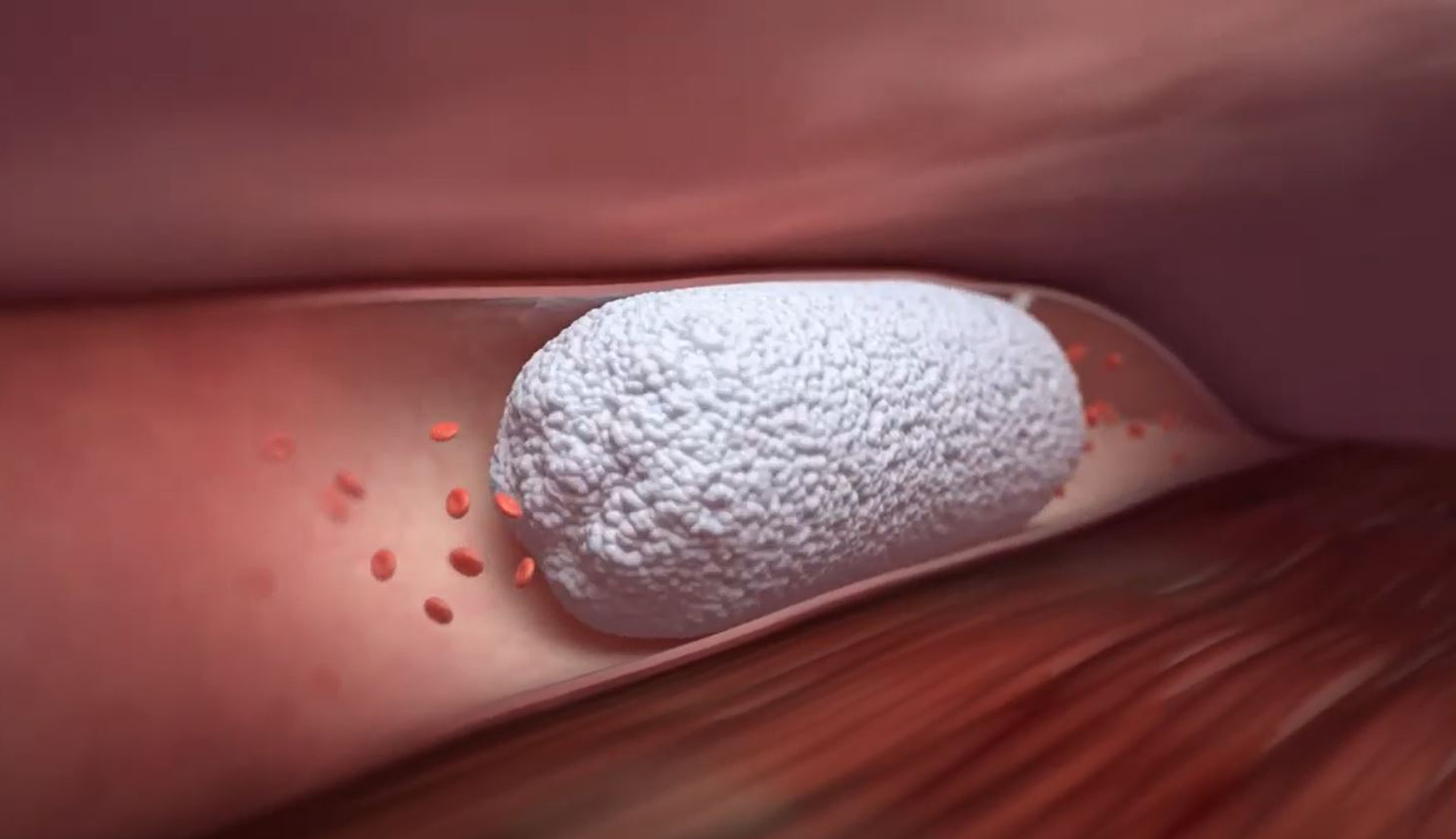
Varithena’s low-nitrogen microfoam comes pre-formulated and delivers a reliably cohesive performance. The small, residual bubbles are rapidly absorbed for consistent results with reduced complications.
- O2:CO2 (65:35) gas mixture with <0.8% nitrogen
- Reliably small bubbles (median diameter <100 µm; all ≤500 µm)
- 7:1 gas: liquid ratio enhances blood displacement to allow for longer dwell time in the vessel
- 1. Incompetent tributaries can be treated through either one injection site, or when warranted, multiple injection sites.
- 2. A column of microfoam advances to fill the vein.
- 3. Varithena displaces blood, effectively filling the lumen for circumferential contact.1
- 4. Varithena achieves endothelial destruction with very low polidocanol concentration.
- 5. The vein contracts, narrowing the lumen until vein has almost no volume.
- 6. Residual, low-nitrogen bubbles are highly absorbable in blood and are swept away and absorbed in venous circulation.
UDSS™ microfoam—the key to consistency.
Specially formulated with patented technology, Varithena produces a uniform density, size, and stability (UDSS™) microfoam that displaces blood in the widest range of vessel shapes and sizes. Gentle and easy-to-deliver, this NTNT solution provides reliable results while reducing complications.
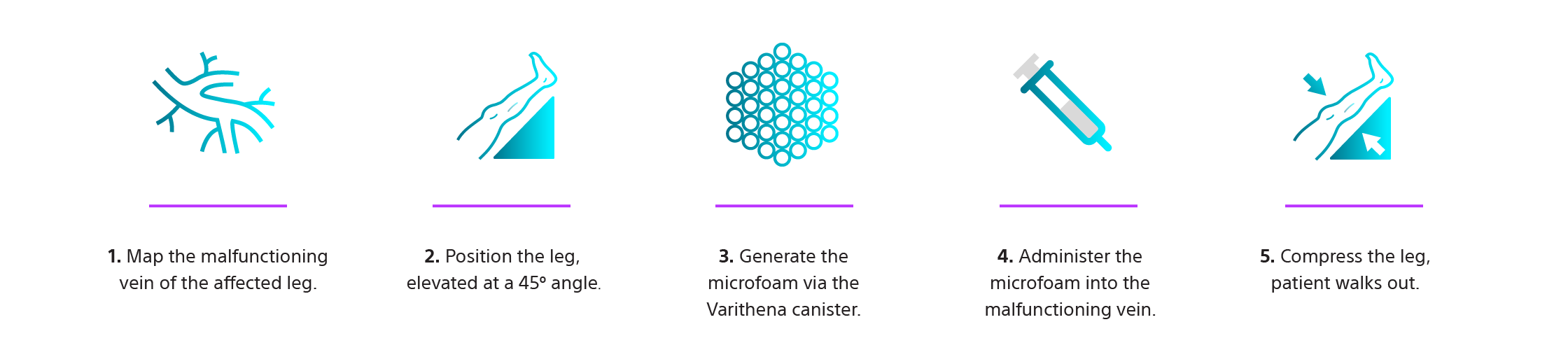
Straightforward Treatment Steps.

Order
Varithena
Add to your practice with this versatile, efficient, and consistent varicose vein treatment. Order directly from Boston Scientific.

Become Varithena certified
Join the 2,000+ certified physicians improving patients’ lives with Varithena. We’re here to help you, and becoming certified is easy.

Contact a sales representative
Have your local Varithena
sales representative
contact you.

Join the physician
finder
Patients want to know who’s performing Varithena procedures. Get noticed by potential patients.
References
1. Eckmann DM. Polidocanol for endovenous microfoam sclerosant therapy. Expert Opin Investig Drugs. 2009 Dec;18(12):1919-27. doi: 10.1517/13543780903376163.
Indications
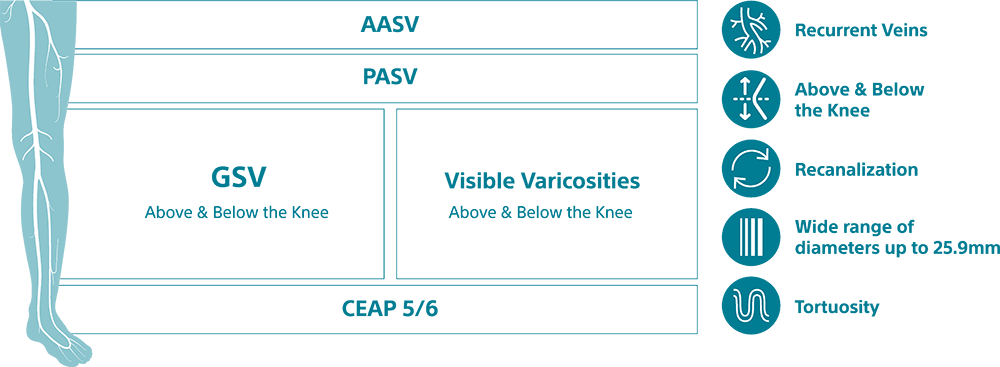
Varithena® (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena® improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
Important Safety Information
The use of Varithena® is contraindicated in patients with known allergy to polidocanol and those with acute thromboembolic disease. Severe allergic reactions have been reported following administration of liquid polidocanol, including anaphylactic reactions, some of them fatal. Observe patients for at least 10 minutes following injection and be prepared to treat anaphylaxis appropriately. Intra-arterial injection or extravasation of polidocanol can cause severe necrosis, ischemia or gangrene. Patients with underlying arterial disease may be at increased risk for tissue ischemia. If intra-arterial injection of polidocanol occurs, consult a vascular surgeon immediately.Varithena® can cause venous thrombosis. Follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization, or pregnancy are at increased risk for developing thrombosis. The most common adverse events observed were pain/discomfort in extremity, retained coagulum, injection site hematoma or pain, common femoral vein thrombus extension, superficial thrombophlebitis, and deep vein thrombosis.Physicians administering Varithena® must be experienced with venous procedures, possess a detailed working knowledge of the use of the duplex ultrasound in venous disease and be trained in the administration of Varithena®.
See Full Prescribing Information for Varithena®
Varithena™ is a registered trademark of Boston Scientific. All other trademarks are property of their respective owners.
PI-1263705-AA